Tetrahedron letters 2016 57 40 4477 4479.
Vinyl epoxide rearrangement.
Regioisomeric vinyl oxiranes can be converted to a single dihydrofuran product using these conditions.
It was originally discovered by ivan nikolaevich nazarov 1906 1957 in 1941 while studying the.
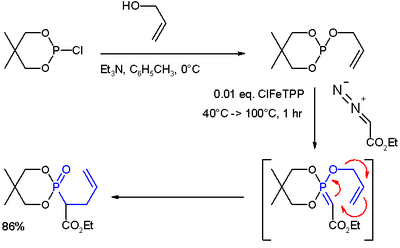
353 354 an oxonium ylide is produced on the attack by the metal carbenoid obtained from the decomposition of a diazo compound equation 94.
Chao hu ren jie song ming hu yuan yang jin heng li shenglian luo.
The aromatic claisen rearrangement is accompanied by a rearomatization.
The rearrangement of acyl nitrenes to isocyanates that is the crux of the hofmann curtius and lossen rearrangements is paralleled by the rearrangement of acyl carbenes to ketenes a transformation called the wolff rearrangement this rearrangement is a critical step in the arndt eistert procedure for elongating a carboxylic acid.
Paréc a département de chimie et biochimie université de moncton moncton nb e1a 3ea canada b instituto de quimica unam 04510 mexico df mexico.
The aliphatic claisen rearrangement is a 3 3 sigmatropic rearrangement in which an allyl vinyl ether is converted thermally to an unsaturated carbonyl compound.
The aza payne rearrangement may be effected in either the forward epoxide to aziridine or reverse aziridine to epoxide direction depending on the conditions employed.
A wide range of vinyl oxiranes can be rearranged to 2 5 dihydrofurans in excellent yields in the presence of electrophilic copper ii acetylacetonate catalysts.
Only two examples have been published on this rearrangement.
Electron poor aziridines undergo the reverse rearrangement in the presence of hydride base 13 while the corresponding epoxy amines undergo the forward rearrangement in.
Spectroscopy 19 2005 171 180 171 ios press brief communication on the mechanism of a new dihalocyclopropane dihalomethyl vinyl rearrangement c k.
A novel copper catalyzed vinyl oxirane ring expansion protocol has been developed.
Rearrangements of acyl carbenes 1.
The reaction of epoxides with allylboranes has been reported only in the context of a concomitant meinwald rearrangement for example cyclopentadiene monoxide derivatives react with allyldialkylboranes to give acyclic trienes equation 151 most likely the reaction occurs through a zwitterionic sigmatropic reaction to give an aldehyde followed by allylation.
Vinyl epoxides can be rearranged in a similar 2 3 fashion via oxonium ylides.
The practical stereocontrolled synthesis of vicinal halohydrins and haloamines from vinyl epoxides and vinyl aziridines.
This method uses low catalyst loadings 0 5 5.