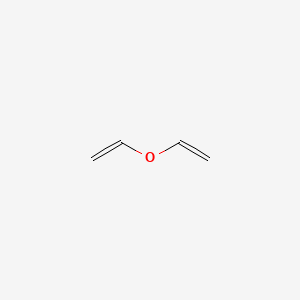
Abstract chlorotrifluoroethylene ctfe and ethyl vinyl ether eve were reacted under radical conditions to produce the poly ctfe co eve alternating copolymer and a full 13 c 1 h and 19 f nmr structural interpretation is offered.
Vinyl ether resonance.
Compound isobutyl vinyl ether with free spectra.
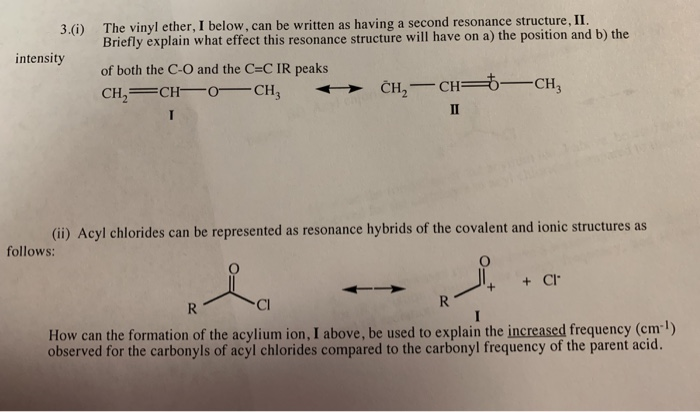
Unlike the other cations the cation produced by protonation of methyl vinyl ether at c 2 is stabilized by resonance.
8 21 227 228 the mechanism for chain transfer is shown in scheme 9 for the case of α benzyloxystyrene 15 the driving force for fragmentation is provided by formation of a strong carbonyl double bond.
Covid 19 is an emerging rapidly evolving situation.
Thus the contributions of the resonance form are greatest in methyl vinyl ether and least in t butyl vinyl ether.
Vinyl ethers 1 x ch 2 a o can be very effective addition fragmentation chain transfer agents.
To determine the reactivity of the acid labile vinyl ether functionality the integral ratio of a distinct vinyl proton resonance of the micelles at 6 49 ppm to the newly appeared proton resonance of acetaldehyde one of the hydrolysis products of the vinyl ether functionality at 9 60 ppm was compared in d 2 o at ph values of 5 0 and 7 4 at 37.
All spectra were characterized by broad signals resulting from the overlapping of different chemical shifts.
On the other hand the resonance in alkyl vinyl ether must lead to a reduction in the olefinic character of the vinyl group and consequently the terminal methyl ene protons will become more equivalent.
Vinyl ether c4h6o cid 8024 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
Methyl vinyl ether is an organic compound with the chemical formula ch 3 och ch 2 a colorless gas it is the simplest enol ether it is used as a synthetic building block as is the related compound ethyl vinyl ether a liquid at room temperature.
As the positive charge develops on the carbon in structure c1 it draws the lone pair of electrons from the adjacent oxygen atom towards itself as indicated by the arrow.
It is also important that r is a good radical leaving group.
Get the latest public health information from cdc.